M. xanthus uses a cascade of enhancer-binding proteins (EBPs) to organize the
transition from exponential growth through the staged development of fruiting bodies
(Caberoy et al. 2003). The cascade of EBPs allows M. xanthus to defer the committ-
ment to sporulation until it has begun to differentiate spores. Though ordinarily
considered an alternative sigma factor, sigma-54 is essential for M. xanthus growth
and development (Keseler, 1997). EBPs are specific transcriptional activators
that work in conjunction with sigma-54 RNA polymerase to activate transcription at
designated sigma-54 promoters (Giglio, 2011). EBPs use the energy from ATP
hydrolysis to form a transcription- competent open promoter complex. Since EBPs
typically activate gene expression in response to a specific interaction with a signal
transduction partner that detects a particular environmental cue, it is suggested that
the cascade's sensor kinases measure the level of metabolites that inform a cell
whether those levels render fruiting body development the outcome to be desired,
despite the cell death that follows. Early detection of approaching starvation seems
to be limiting spore formation and fewer than 1% of the cells initiating fruiting body
development ever become spores (Harvey et al., 2013), It seems that 99% of cells
are cannibalized by the survivors to sustain their continued movement and capsule
synthesis.
In parallel with the starvation-signal-induced cascade of EBPs, the transition from
growth to development is guided by a diffusible cell-to-cell signal, the A-signal. A-signal
molecules, purified from medium conditioned by developing cells, is a set of amino
acids and peptides containing those amino acids (Kuspa, 1986), (Kuspa, 1992).
Each developing Myxococcus cell releases a small quantity of A-signal about
two hours into development, and so the extracellular concentration of A-signal is
directly proportional to the density of M. xanthus cells that have opted to develop.
Cells respond to A-signal only if its concentration is above a certain threshold. The
threshold number of cells constitute a quorum, and A-signal can be considered a
quorum sensor.
After aggregation, M. xanthus cells express a unique set of A-signal-dependent genes,
2 of which are csgA, the gene for the C-signal, and fruA, an important developmental
response regulator.
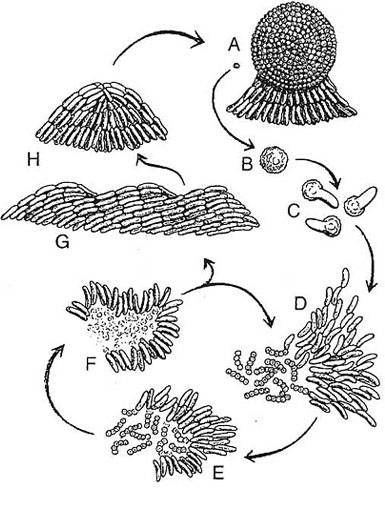
A sketch showing the lifecycle of M. xanthus. A swarm
(a group of moving and interacting cells) can have either
of two fates depending on their environment. The fruiting
body (A) is a spherical structure of ~1x105 cells that have
become stress-resistant spores (B). The fruiting body is
small (0.10 mm high) and sticky, and its spores are tightly
packed. When a fruiting body receives nutrients, the individual
spores germinate (Q and thousands of M. xanthus cells
emerge together as an "instant" swarm (0). When prey is
available (micrococci in the figure), the swarm becomes a
predatory collective that surrounds the prey. Swarm cells
feed by contacting, lysing, and consuming the prey bacteria
(E and F). Fruiting body development is advantageous given
the collective hunting behavior. Nutrient-poor conditions elicit
a unified starvation stress response. That response initiates
a self-organized program that changes cell movement behavior,
leading to aggregation. The movement behaviors include
wave formation (G) and streaming into mounded aggre¬gates
(H), which become spherical (A).

During the aggregation of M. xanthus cells into fruiting
bodies, dense ridges of cells appear to move in traveling
waves called ripples. Both fruiting body development
and rippling are observed in various myxobacterial
species.